--- CHEMIST 87 ---
شیمی برای ما؛ شیمی برای زندگی--- CHEMIST 87 ---
شیمی برای ما؛ شیمی برای زندگیحجم فلزات
تمامی فلزات بجز آنتیموان و بیسموت در مواقع انجماد حجمشان کاهش می یابد!
acetaminophen استامینوفن
The drug acetaminophen is a pain reliever (an analgesic ) and a fever- reducing agent (an antipyretic). It is found in over-the-counter medicines such as Tylenol and Excedrin. It is widely used to treat both chronic and acute pain and is considered to have a pain-relieving potency similar to that of other over-the-counter analgesics, such as aspirin and ibuprofen. Its chemical name is 4-hydroxyacetanalide. Its chemical formula is C 8 H 9 NO 2 (see Figure 1).
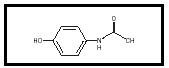
Acetaminophen was used as a pain reliever as early as the late 1800s. It was approved for use by the U.S. Food and Drug Administration in 1950, shortly after it was discovered that the closely related drug paracetin was broken down in the body to acetaminophen, and that the beneficial effects of paracetin were actually the effects of acetaminophen.
Acetaminophen works by inhibiting the synthesis of chemical messengers called prostaglandins, which help to transmit pain signals and induce fever. The body produces prostaglandins in response to an injury or illness. Acetaminophen reduces the pain by helping to block this signaling. Acetaminophen stops some prostaglandin functions while not affecting others. Prostaglandins are known to promote inflammation and swelling of many body tissues. Unlike aspirin and ibuprofen, acetaminophen does not have anti-inflammatory action.
The differences in the actions of these drugs involve their tissue specificities. Aspirin and ibuprofen act on a broad range of tissues. Acetaminophen inhibits prostaglandin synthesis more specifically in the cells of the nervous system and is a much less effective inhibitor of this in other tissues. This selectivity gives acetaminophen its analgesic and antipyretic effects without acetaminophen's acting as an anti-inflammatory drug.
Acetaminophen is known to cause less stomach irritation than aspirin and ibuprofen, and it does not inhibit platelet aggregation and blood clotting (as does aspirin).
When given in its therapeutic dose (500 mg every 4–6 hours), acetaminophen is a safe and effective pain reliever. However, at higher doses it can be severely toxic to the liver, and even fatal.
The drug itself is not toxic, but a toxic compound, N-acetyl- p -benzoquinonimine, is formed from it as it is broken down by enzymes in the liver. In small amounts this compound can be detoxified and excreted . But in large amounts it overwhelms the detoxification system and the compound begins killing liver tissue. Overdose can be treated by giving the patient activated charcoal, which absorbs the acetaminophen in the patient's stomach and intestines, and by administering N -acetylcystine, a compound that can deactivate the toxic product of metabolism .
Uranium
خصوصیتهای قابل توجه
اورانیوم هنگام عمل پالایش به رنگ سفید مایل به نقره ای فلزی با خاصیت رادیو اکتیوی ضعیف می باشد که کمی از فولاد نرم تر است. این فلز چکش خوار ، رسانای جریان الکتریسیته و کمی PARAMAGNETIC می باشد. چگالی اورانیوم 65% بیشتر از چگالی سرب میباشد. اگر اورانیوم به خوبی جدا شود به شدت از آب سرد متاثر شده و در برابر هوا اکسید میشود. اورانیوم استخراج شده از معادن می تواند به صورت شیمیایی به دی اکسید اورانیوم و دیگر گونه های قابل استفاده در صنعت تبدیل شود.
اورانیوم در صنعت سه گونه دارد:
• آلفا (ORTHOHOMBIC) که تا دمای 667.7 درجه پایدار است.
• بتا (TETRAGONAL) که از دمای 667.7 تا 774.8 درجه پایدار است.
• گاما (BODY-CENTERED CUBIC) که از دمای 774.8 درجه تا نقطه ذوب پایدار است. ( این رساناترین و چکش خوارترین گونه اورانیوم می باشد.)
دو ایزوتوپ مهم ان
ادامه مطلب ...